FDA Approves Twice A Year HIV Injections That Block Infection At 96% Success, But Funding Could Make It Hard To Access

FDA Approves Twice A Year HIV Injections That Block Infection At 96% Success, But Funding Could Make It Hard To Access
The FDA has approved Yeztugo, a powerful twice-yearly injection that nearly eliminated HIV transmission in clinical trials.
Made by Gilead Sciences, Yeztugo—also known as lenacapavir—offers a new option for those unable to stick to daily pills like Truvada.
“This is the single best opportunity in 44 years of HIV prevention,” said AVAC’s Mitchell Warren.
Studies show Yeztugo reduced HIV rates by up to 96% in high-risk groups, but its success faces major obstacles.
At $14,000 per shot and amid proposed Trump-era budget cuts—like eliminating the CDC’s HIV-prevention division and reducing NIH research funding—advocates worry the drug won’t reach the communities who need it most.
Experts warn that high costs, insurance pushback, and access issues could worsen disparities, especially for Black and Latino gay men. Still, Gilead plans to cover costs for uninsured patients and push for broad coverage. The rollout could be a turning point—if the system supports it.
What are your thoughts on this medical breakthrough?


 Previous Article
Previous Article Next Article
Next Article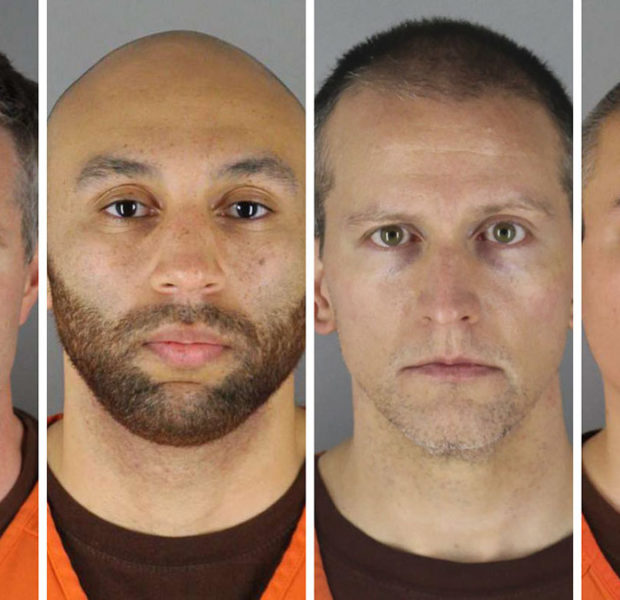 George Floyd – Officers Involved With His Death Blame Each Other, Now Seeking Separate Trials
George Floyd – Officers Involved With His Death Blame Each Other, Now Seeking Separate Trials ![Kevin Hart Blames Himself For Ruining 1st Marriage To Torrei Hart: I take responsibility. [VIDEO]](https://thejasminebrand.com/wp-content/uploads/2016/10/Kevin-Hart-Admits-He-Messed-Up-1st-Marriage-To-Torrei-Hart-the-jasmine-brand.jpg) Kevin Hart Blames Himself For Ruining 1st Marriage To Torrei Hart: I take responsibility. [VIDEO]
Kevin Hart Blames Himself For Ruining 1st Marriage To Torrei Hart: I take responsibility. [VIDEO]  American Airlines Cracks Down On People Who Cut The Line to Board Faster, Known As ‘Gate Lice’
American Airlines Cracks Down On People Who Cut The Line to Board Faster, Known As ‘Gate Lice’  Kevin Clash’s Second Underage Accuser Gives Intimate Details + What’s Gonna Happen to Elmo?
Kevin Clash’s Second Underage Accuser Gives Intimate Details + What’s Gonna Happen to Elmo?  Birdman, Dame Dash, Jermaine Dupri & Snoop Star in New Reality Show, ‘Music Moguls’
Birdman, Dame Dash, Jermaine Dupri & Snoop Star in New Reality Show, ‘Music Moguls’ 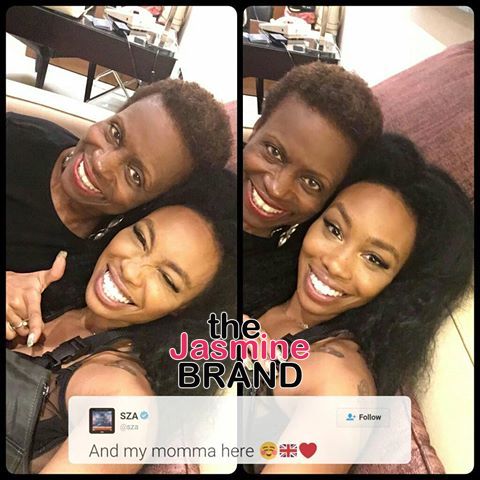 SZA’s Mom Gives Us The Advice That We All Need
SZA’s Mom Gives Us The Advice That We All Need  Celebrity Stalking: Jay Z, Rachel Roy, Wesley Snipes, Iggy Azalea, Yolanda Adams, Jordin Sparks
Celebrity Stalking: Jay Z, Rachel Roy, Wesley Snipes, Iggy Azalea, Yolanda Adams, Jordin Sparks  Michelle Williams Says She’s ‘Better’ After Checking Herself Into Mental Health Facility, Posts Message To Fans
Michelle Williams Says She’s ‘Better’ After Checking Herself Into Mental Health Facility, Posts Message To Fans